Legalizing abortion does not guarantee that those who need this vital service can get it. For many people who want to end a pregnancy, their path is blocked by long travel times, stigma, threat of violence, or expensive fees for surgical methods. Abortion with pills allows them the freedom to take care of this acute need in their home or another secure space. That’s why the fight for self-managed abortion is a crucial aspect of reproductive justice.
When it comes to access, abortion with pills is a game changer—as long as we make safe, effective abortion medication accessible.
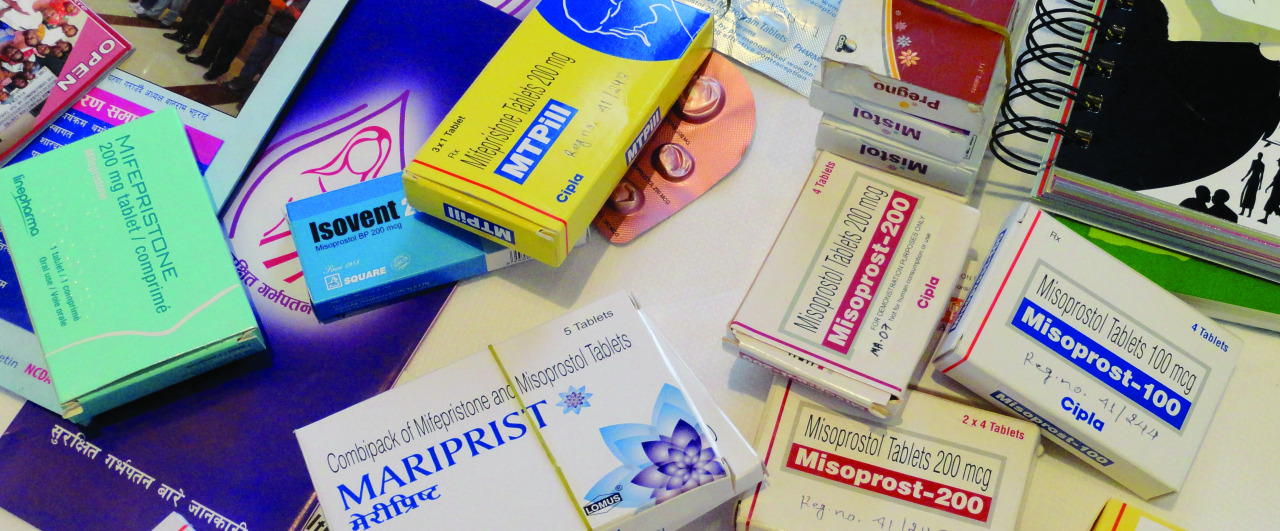
Ipas works with local governments and established health systems to normalize self-managed abortion in communities and to ensure that providers and retailers offer help, information and quality products without stigma. Ensuring the availability of quality abortion medicines is a powerful win for access, and the first step in doing so is getting abortion medications (mifepristone and misoprostol) registered on the essential medicines list within a country. This helps that country’s government ensure the registered medicine is safe, effective and of acceptable quality, making it available for distribution through government-vetted regional retailers.
Both Ipas Nigeria and Ipas Democratic Republic of Congo (DRC) have achieved such a win for their regions through their tireless work and commitment towards the registration of abortion medications on the Essential Medicines List, earning recognition as key partners in expanding abortion access. Mifepristone was registered in Nigeria in 2020 (2016 for Misoprostol). The DRC also registered mifepristone in 2020 but it was only available in health facilities, until the Ministry of Health authorized distribution in pharmacies in September of 2021.
Registering these medicines didn’t happen overnight though. What can past success teach us about registering these medicines in other countries?
Dr. Ernest O Nyamato, Ipas associate director of Quality of Care, explains that Ipas teams and partners “use an evidence-driven, collaborative and structured approach. Developing standards and guidelines, advocating for inclusion of mifepristone in the essential drug list, using the Maputo Protocol (DRC) and working closely with the ministries of health.”
Jean-Claude Mulunda, director of Ipas DRC, says the registration of mifepristone in the DRC “allows this vital ingredient of abortion self-management to be available in the country and accessible through pharmacies. Its availability and accessibility empower women to take a central role in managing their own health.”
Lucky Palmer, Ipas Nigeria director, echoes Mulunda in his assertion that registration truly increases access. “In Nigeria, the listing of mifepristone has removed a critical barrier to accessing safe and efficient abortion drugs because government health facilities in remote villages can now procure and store the drug,” he says. “It will increase access to essential services through cost reduction—and will potentially save lives.”